Synonyms
β-Nicotinamide adenine dinucleotide, reduced disodium salt hydrate; β-DPNH; β-NADH; DPNH; Diphosphopyridine nucleotide, reduced form; β-DPNH; Coenzyme-I
Technical Data
CAS Number: 606-68-8
Molecular Formula: C21H27N7Na2O14P2
Molecular Weight: 665.4 (anhydrous free acid); 709.4 (disodium anhydrate); 763.5 (disodium trihydrate)
EC Number: 210-123-3
FabriChem Specifications
| ITEMS | SPECIFICATIONS |
| Purity | ≥ 95.0% |
| NADH Contents | - |
| Sodium Contents | 6.5 ± 1.5% |
| Water Contents | < 8.0% |
| Spectral Analysis (at pH 10): | |
| ε at 260 nm | (14.4 ± 0.5) x 103 L/mole/cm |
| ε at 340 nm | (6.3 ± 0.2) x 103 L/mole/cm |
| Ratio (at pH 10): | |
| Ratio A250/ A260 | 0.82 ± 0.03 |
| Ratio A280/ A260 | 0.23 ± 0.02 |
| Ratio A340/ A260 | 0.43 ±0.01 |
| |
Molecular Structure
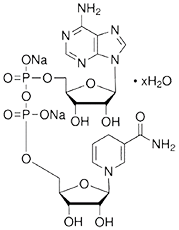
About
Nicotinamide Adenine Dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.
In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD.
Compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.
[/columns2]
